Alpha Decay
Alpha Decay
This lesson aligns with NGSS PS1.C
Introduction
This natural process involves the emission of an alpha particle, a cluster of two protons and two neutrons, from an unstable atomic nucleus. The particle expelled during this process is commonly referred to as an alpha particle. Ernest Rutherford studied the deflection of radiation in a magnetic field, and differentiate alpha decay from other radiation types. The deflection observed in alpha decay is characterized by a positive charge, attributed to the fact that the alpha particles possess a charge of +2e. The purpose of this article is to learn about alpha decay, exploring its characteristics, mechanisms, and providing real-world examples to enhance our understanding.
Understanding Alpha Decay
Alpha decay, denoted as α-decay, represents a specific category of radioactive decay where the atomic nucleus expels an alpha particle, leading to the transformation or decay into a new atomic nucleus.
Nature of Alpha Particles:
Alpha particles, denoted as α, are helium-4 nuclei (He). These particles are relatively massive compared to other forms of nuclear radiation, making them unique in the decay process. The emission of an alpha particle results in a decrease of two protons and two neutrons from the original nucleus.
Characteristics of Alpha Decay:
Alpha decay is primarily observed in heavy, neutron-rich atomic nuclei. Unstable nuclei undergo alpha decay as a means of achieving a more balanced and stable configuration. The process is governed by the principle of seeking a lower energy state, and alpha particles are emitted to achieve this stability.
Mechanism of Alpha Decay:
The mechanism of alpha decay involves quantum tunneling through the potential barrier of the nucleus. Despite the repulsive forces between positively charged protons, alpha particles can "tunnel" through the potential barrier and escape the nucleus. This phenomenon is a consequence of the probabilistic nature of quantum mechanics.
Energy Release:
One notable aspect of alpha decay is the release of energy. As the alpha particle escapes the nucleus, the parent nucleus transforms into a daughter nucleus, resulting in the release of kinetic energy and the alpha particle itself. The emitted alpha particle carries a specific energy characteristic to the decay.
Real-world Examples:
Uranium-238 to Thorium-234:
A classic example of alpha decay is the transformation of uranium-238 into thorium-234
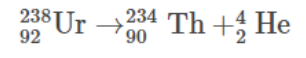
In this process, the uranium nucleus emits an alpha particle, leading to a reduction in two protons and two neutrons. This conversion marks the beginning of a decay chain that eventually results in the formation of stable lead isotopes.
Radon-222 Decay:
Radon-222 is another example of an alpha-emitting radionuclide. Radon is a colorless, odorless gas that naturally occurs from the decay of uranium and thorium in the Earth's crust. During the alpha decay of radon-222, the emitted alpha particle transforms it into polonium-218.

Radium-226 in Uranium Decay Series:
Radium-226 is part of the uranium decay series and undergoes alpha decay to radon-222. This radium isotope was historically used in luminescent paint, contributing to the glow of watch dials. However, due to its radioactivity, safety concerns led to the discontinuation of this practice.

Gamow Theory of Alpha Decay
The Geiger–Nuttall law, also known as the Geiger–Nuttall rule, establishes a correlation between the decay constant of a radioactive isotope and the energy of the emitted alpha particles. Additionally, this rule states that half-lives exhibit an exponential dependence on decay energy. Consequently, significant variations in half-life result in relatively minor differences in decay energy and, by extension, alpha particle energy.
According to this principle, isotopes with shorter lifespans release alpha particles with higher energy compared to their longer-lived counterparts. The formulation of this law is attributed to the work of Hans Geiger and John Mitchell Nuttall in 1911, leading to its eponymous recognition in their honour.
Importance of Alpha Decay in Nuclear Physics:
Nuclear Stability:
Unstable nuclei with excess protons and neutrons undergo alpha decay to achieve a more balanced nuclear configuration.
Decay Chains:
Alpha decay is often a precursor to more complex decay chains. As one nucleus undergoes alpha decay, the resulting daughter nucleus may further decay through different processes, contributing to the natural radioactivity of certain elements.
Cosmic-Ray Interactions:
Understanding alpha decay is essential for comprehending cosmic-ray interactions with matter. High-energy cosmic rays can induce nuclear reactions, including alpha decay, in the Earth's atmosphere and other celestial bodies.
Summary
- The alpha decay process involves the emission of an alpha particle, a cluster of two protons and two neutrons, from an unstable atomic nucleus.
- The emission of an alpha particle results in a decrease of two protons and two neutrons from the original nucleus.
- As the alpha particle escapes the nucleus, the parent nucleus transforms into a daughter nucleus, resulting in the release of kinetic energy and the alpha particle itself.
Related Worksheets:













